Our Research
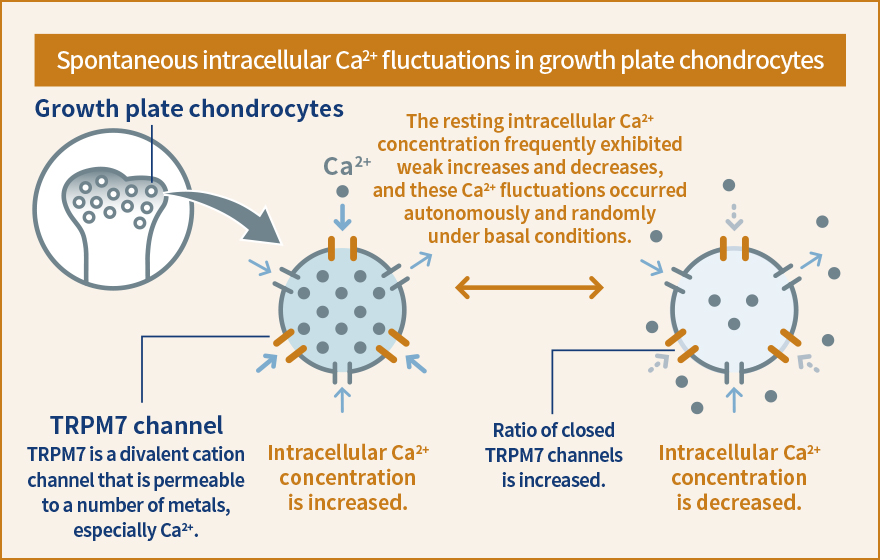
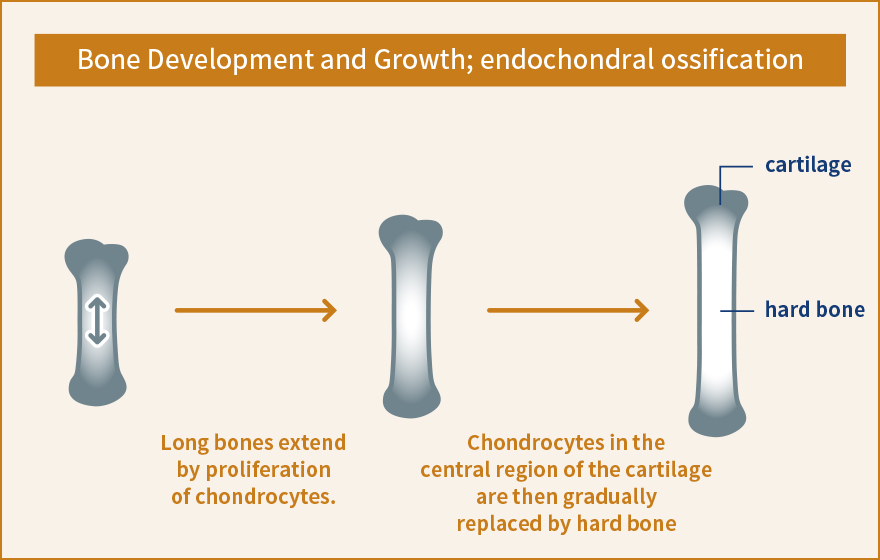
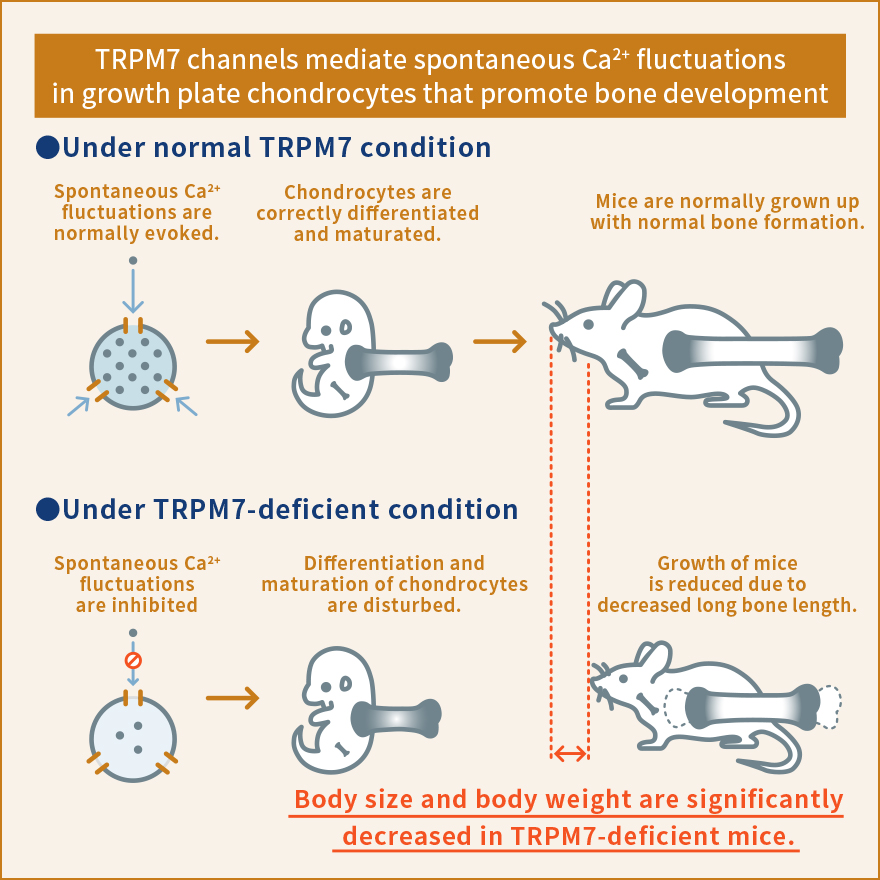
The fatty acid receptor is one of the molecules that have recently been found to act as a stress response sensor. Fatty acids were believed to be merely a source of energy, but were discovered to transmit various signals into a cell by binding to fatty acid receptors. The signals are likely to regulate the metabolism of the whole body upon exposure to stress, such as excessive intake of energy and starvation, by inducing physiological functions such as 1) stimulating secretion of insulin, GLP-1 or another peptide hormones that control blood sugar level and induce take up of glucose from the blood, and 2) storing fat by stimulating the differentiation and maturation of adipocytes.
I have mainly worked on GPR120, a receptor of mid- to long-chain fatty acids including α-linolenic acid and DHA. When knockout mice that lacked the GPR120 gene were fed a high fat diet, the mice showed increases in the weight of the liver and white fat. From this, we found that the GPR120 knockout mouse was prone to gaining weight from a high fat diet and developing abnormal carbohydrate metabolism. The same was also found to apply to humans. In a French international joint study that involved examining the genome of about 10,000 obese and non-obese individuals, the obese group was found to have a higher probability of having a mutation of GPR120 compared to the non-obese group. The data suggests that persons with a genetic loss of mutation in GPR120 have a tendency to gain weight easily.
I am planning to perform a precise analysis on how GPR120 and a series of other fatty acid receptors, whose functions in each organ have not been unveiled, control the energy metabolism of the whole body by using mice that lack the genes only in specific single organs. Elucidation of these molecular mechanisms and control by using low-molecular weight compounds are expected to lead to the development of new drugs for metabolic diseases such as obesity and diabetes.
I have mainly worked on GPR120, a receptor of mid- to long-chain fatty acids including α-linolenic acid and DHA. When knockout mice that lacked the GPR120 gene were fed a high fat diet, the mice showed increases in the weight of the liver and white fat. From this, we found that the GPR120 knockout mouse was prone to gaining weight from a high fat diet and developing abnormal carbohydrate metabolism. The same was also found to apply to humans. In a French international joint study that involved examining the genome of about 10,000 obese and non-obese individuals, the obese group was found to have a higher probability of having a mutation of GPR120 compared to the non-obese group. The data suggests that persons with a genetic loss of mutation in GPR120 have a tendency to gain weight easily.
I am planning to perform a precise analysis on how GPR120 and a series of other fatty acid receptors, whose functions in each organ have not been unveiled, control the energy metabolism of the whole body by using mice that lack the genes only in specific single organs. Elucidation of these molecular mechanisms and control by using low-molecular weight compounds are expected to lead to the development of new drugs for metabolic diseases such as obesity and diabetes.